DEVICES
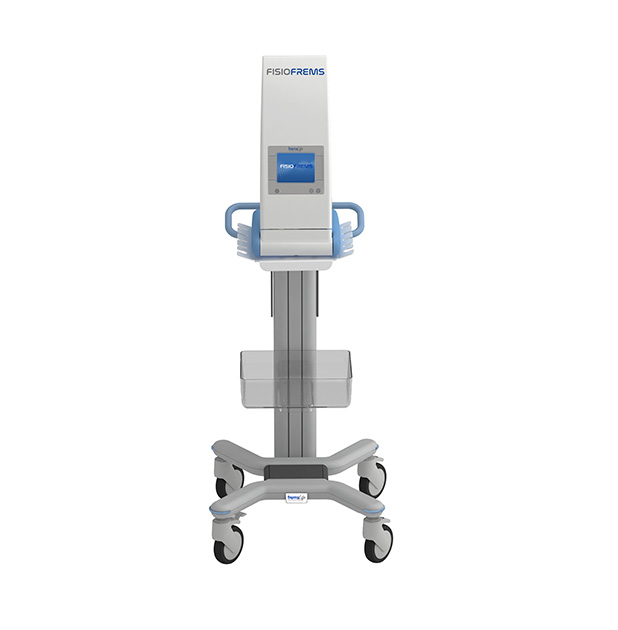
APTIVA FISIO FREMS™
The new Medical Device APTIVA FISIO FREMS ™ is dedicated to the treatment of pathologies affecting the musculoskeletal system.
It represents the evolution of a technology consolidated over time that has already demonstrated its high therapeutic efficacy. With the improvements made, the ease of use and the safety of the results have increased.
OPERATOR INDEPENDENT
SCIENTIFICALLY SUPPORTED
WIDE RANGE OF APPLICATIONS
NO SIDE EFFECTS
EASY TO USE
ONGOING TRAINING
FEATURES
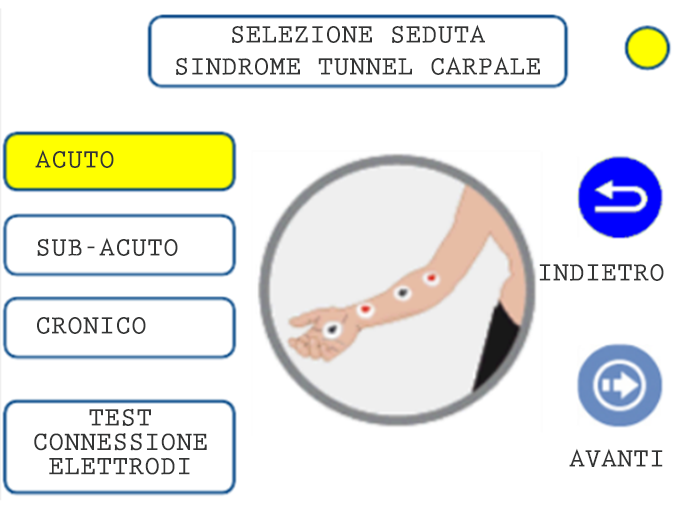
It is equipped with 2 independent neuro stimulation channels each of which is equipped with 4 dipoles. The 16 electrodes therefore allow an adequate differentiated stimulation capacity for the over 80 application protocols. For each treatment, three different sessions are available: Acute, Sub-acute, Chronic.
It is possible to navigate within the menu thanks to a touch screen interface and a remote control.
It is also equipped with the electrode detachment control function which checks if the electrodes are correctly transmitting FREMS ™ therapy, in order to optimize the final result.
INTERFACCIA USER-FRIENDLY
An intuitive organization of the navigation menu allows you to reach the desired treatment in a few clicks.
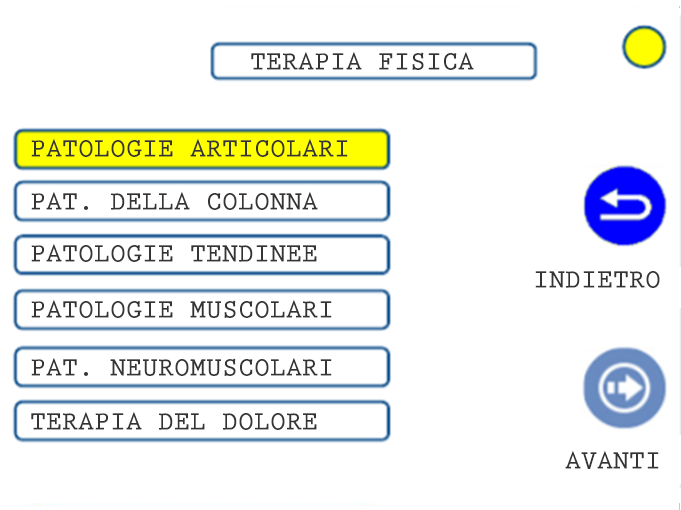
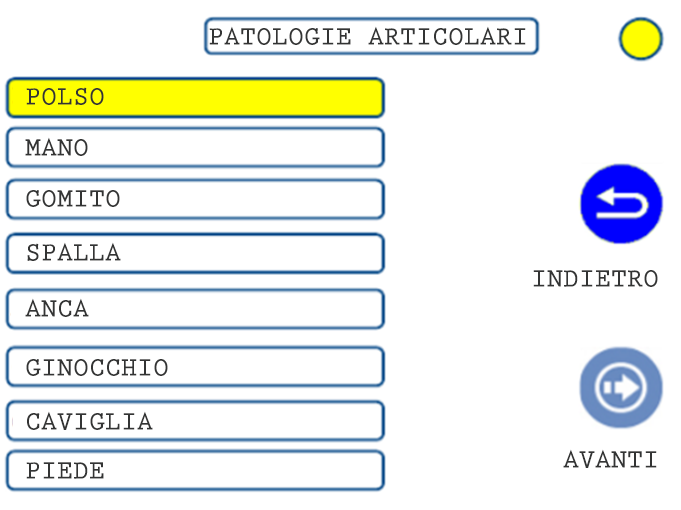
01. TREATMENT SELECTION: CLINICAL AREA
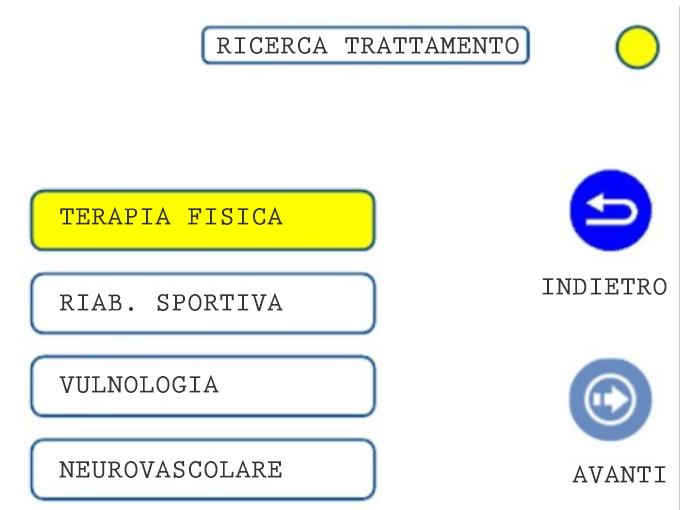
04. TREATMENT SELECTION
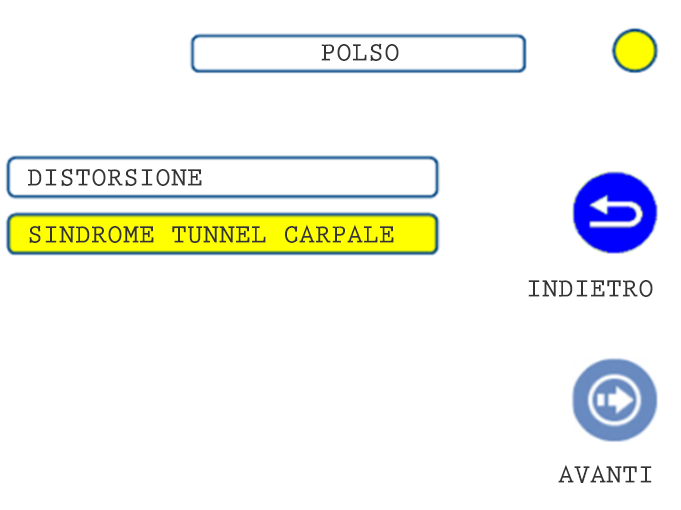
02. TREATMENT SELECTION: PATHOLOGY
05. SESSION SELECTION
03. TREATMENT SELECTION: PATHOLOGY 2
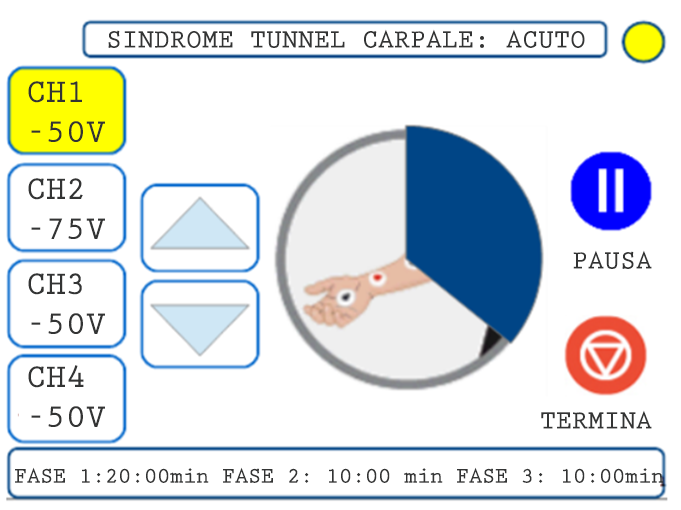
06. EXECUTION OF THE TREATMENT
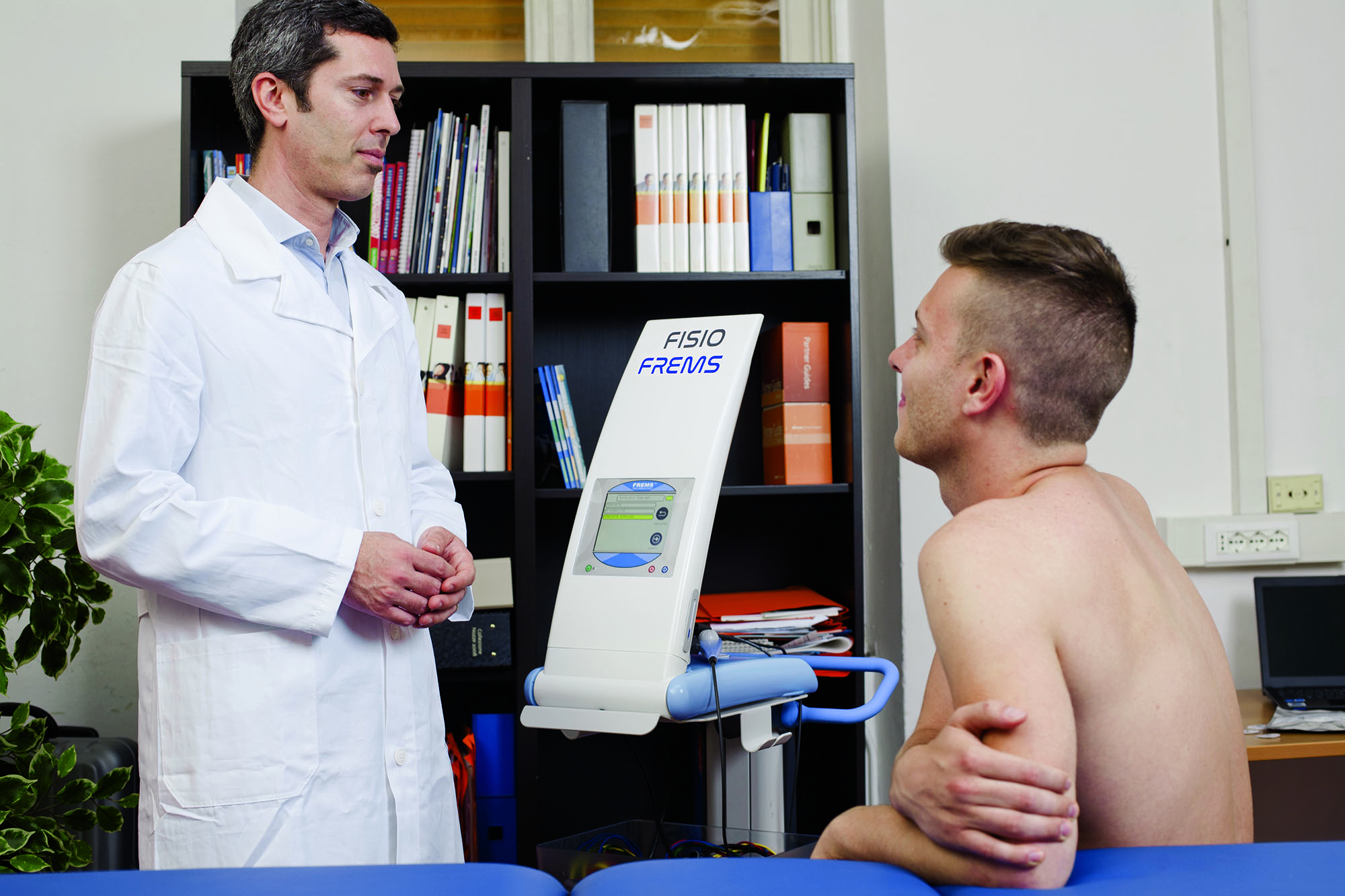
HOW TO APPLY
Frems™ consists in the application of an electrical signal that is transmitted through transcutaneous electrodes. These electrodes are dedicated and specific for the Frems ™ application and must be positioned according to rules defined for each specific treatment and protocols tested for each individual application. The treatment consists of a cycle of daily sessions of about 30 minutes to be carried out on an outpatient basis or at home for a period of a few weeks.


The treatment is administered by placing transcutaneous electrodes with a reduced contact surface, designed and certified specifically for Frems™ applications, on the area to be treated.
Two application manuals, one for PHYSICAL THERAPY and the other for SPORTS REHABILITATION, indicate the correct positioning of the electrodes and the duration of each treatment.
PROTOCOL EXAMPLE: CARPAL TUNNEL SYNDROME
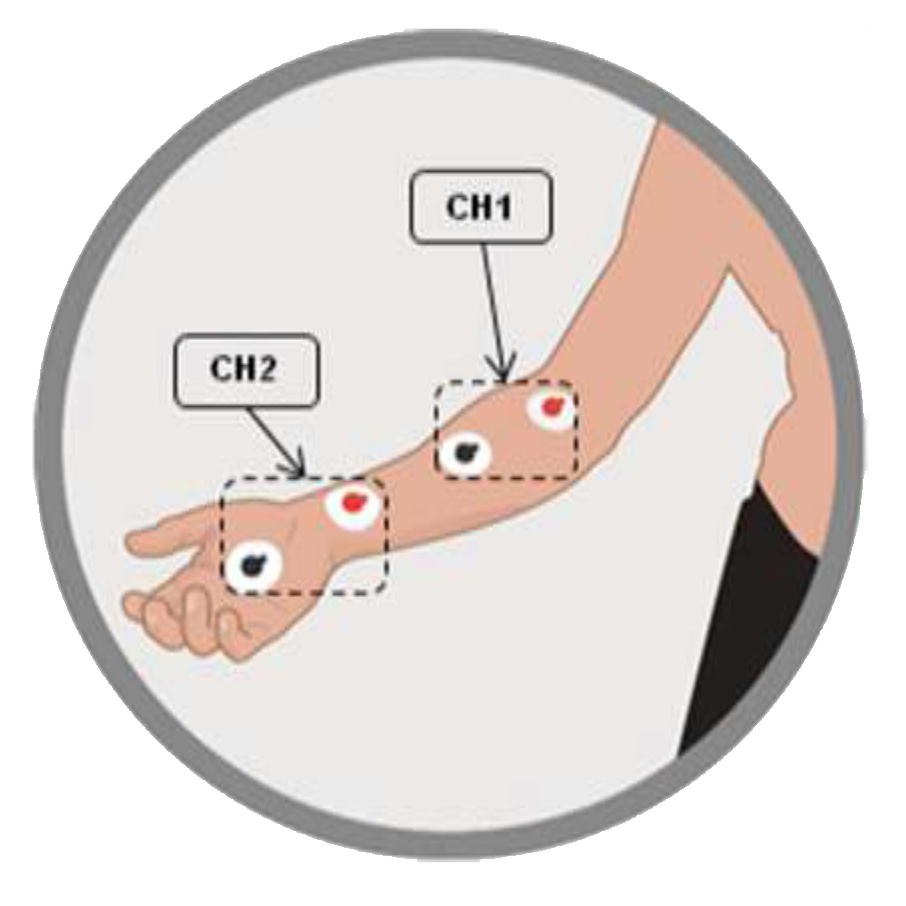
Channel 1 (CH1) positioning
Place a pair of electrodes on the forearm, in correspondence with the course of the median nerve, with the red one in the proximal position
Channel 2 (CH2) positioning
Place a pair of electrodes on the wrist joint, with the red one proximal. In case of bi-lateral treatment, follow point 10 of the application standards
Possibility of bilateral treatment
In case of bilateral treatment, repeat the same pattern on the other limb
Duration of treatment
ACUTE SESSION: 26 min
SUB-ACUTE SESSION: 26 min
CHRONIC SESSION: 36 min
APPLICATIONS VIDEO
LUMBOSCIATALGIA
ACUTE SHOULDER
CERVICALGIA
DISTORTION
MUSCLE INJURIES
TENDINOPATHY
CARPAL TUNNEL
ELECTRODES
They represent an essential component to ensure the effectiveness of the treatment.
The contact impedance between electrode and patient represents a potential criticality for energy transfer. The original Frems ™ electrodes have been specifically designed to optimize this energy transfer.
The low contact surface has been specially designed so that, together with the biocompatible material with which it is made, the best impedance is guaranteed for the greatest energy transfer.

APTIVA FISIO FREMS ™ is a medical device (class IIa) with the CE and INMETRO mark. The equivalent APTIVA MOVE model, whose sale is limited to Russia, bears the GOST R trademark. Software, user manuals and application manuals are available in Italian, English, Spanish, Portuguese and Russian.
In order to increase the manageability and practicality of the device, it is possible to request a transport case and a trolley on which to install the device, thus being able to transport it easily in an outpatient environment and being able to store the stimulation cables in an orderly manner.
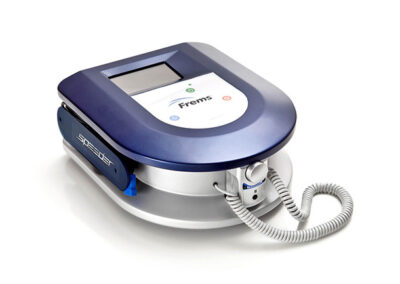
speeder FREMS™
PORTABLE DEVICE
Speeder is the FREMS ™ tool ready for use in any situation, thanks to its portability and easy handling. It has a compact design and uses a touch screen interface and soft keys keyboard.
It features two stimulation channels (each of which has two dipoles) and uses FREMS ™ branded disposable electrodes.
The software is intuitive and contains protocols for physical therapy and sports. For each treatment, three different sessions are available: Acute, Sub-acute, Chronic.
The following classes of patients are excluded from the use of APTIVA FISIO FREMS and SPEEDER:
- patients with diagnosed neoplastic pathology;
- patients with an implantable electronic device (e.g. pacemaker, defibrillator or neurostimulator);
- pregnant women;
- patients with previous seizure events.
ARTICLES AND PAPERS
We have demonstrated, with the scientific evidence given by about 100 national and international scientific studies, that the results obtained with the Frems ™ technology represent an effective solution for the treatment of many diseases of the musculoskeletal system.
TRAINING
Various training opportunities, which meet every specific need: one-to-one meetings with one of our trainers, webinars, video tutorials and ECM courses.
ContaCT US
To request more information, complete the form and we will forward the request to the competent office and contact you as soon as possible.

WHO
Fremslife S.r.l.
P.I. 02329170993
WHERE
Via Buccari, 9
16153 – Genoa, Italy
CONTACT
T: (+39) 010 6402 911
F: (+39) 010 6402 900
WA: (+39) 375 6476512
info@fremslife.com
Pursuant to the guidelines of the Ministry of Health relating to health advertising concerning medical devices, users of this website are informed that the information is intended exclusively for professional operators. Seek advice from a qualified medical professional if you believe you have a medical condition discussed on our site.